Atrophic rhinitis
Inspectors reported lesions consistent with atrophic rhinitis, but the aim of distinguishing between progressive and non-progressive atrophic rhinitis. Both processes are of a multifactorial etiology and with morphologically similar lesions but the pathological significance is different.
|
Nonprogressive atrophic rhinitis |
Progressive atrophic rhinitis |
|
|
Etiology |
Bordetella bronchiseptica |
Pasteurella multocida (dermonecrotic toxin positive). |
|
Predisposing factors |
Poor air quality (dust, ammonia...). |
Concurrent B.bronchiseptica infection Poor air quality (dust, ammonia...). |
|
Lesions |
Snout distortion. Atrophy and malformation of nasal turbinates. Brachyignathia superior (shortening of the maxillary bone). |
|
|
Diagnosis |
Microbiological culture of B. bronchiseptica from nasal swabs samples. |
Determine the presence of dermonecrotic toxin positive - P.multocida Microbiological culture from nasal swabs or via direct nasal swab PCR. |
|
Observations |
Limited pathological significance. |
Might cause significant production losses. Export restrictions. |
For diagnosis in slaughterhouse animals the ideal sample are nasal swabs taken before scalding. But tissue samples (nasal turbinates) of scalded and/or frozen carcasses can also be submitted for direct PCR identification of P.multocida and its toxin. nasal swabs without transport medium can be used, ensuring contact with the nasal mucosa, and it is best to send them at room temperature as the cold can inactivate the pasteurella. In case of late shipping, it is recommended to freeze the swabs.
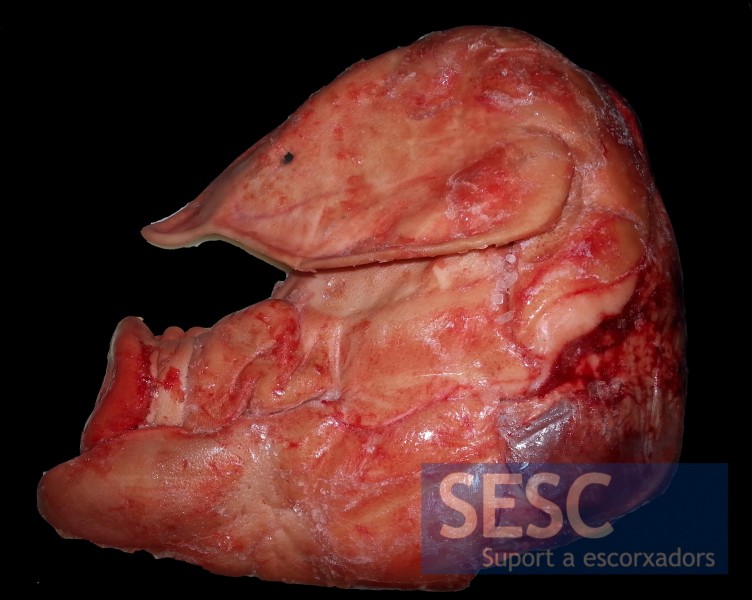
Pig skull with shortened snout (brachyignathia). The presence of P. multocida could not be confirmed with the direct PCR.
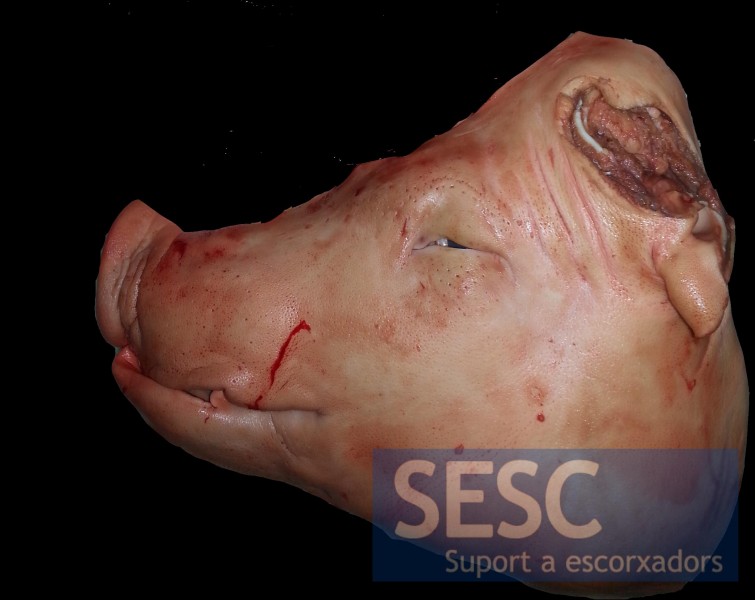
Another case of a pig carcass with distorted snout. In this case, direct PCR revealed the presence of dermonecrotic toxin positive P. multocida.
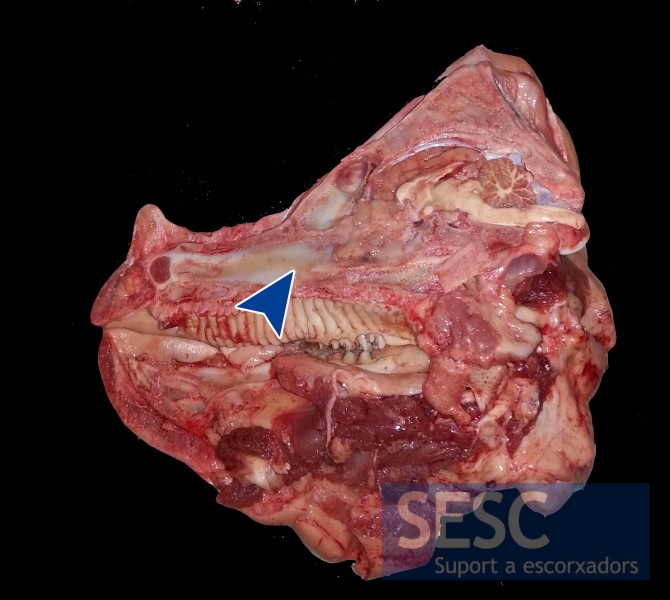
Longitudinal section of the skull, complete turbinate atrophy can be appreciated. In this case PCR revealed the presence of dermonecrotic toxin positive P. multocida.
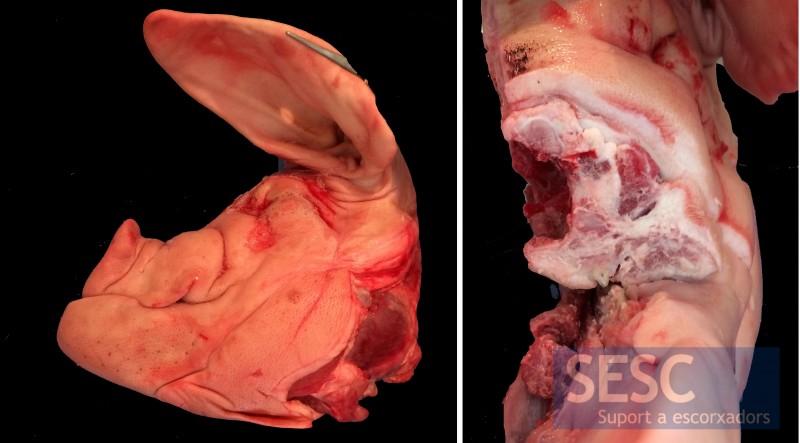
Another case of pig skull with shortened snout (brachyignathia) and, in the cross section, atrophy of the nasal turbinates.
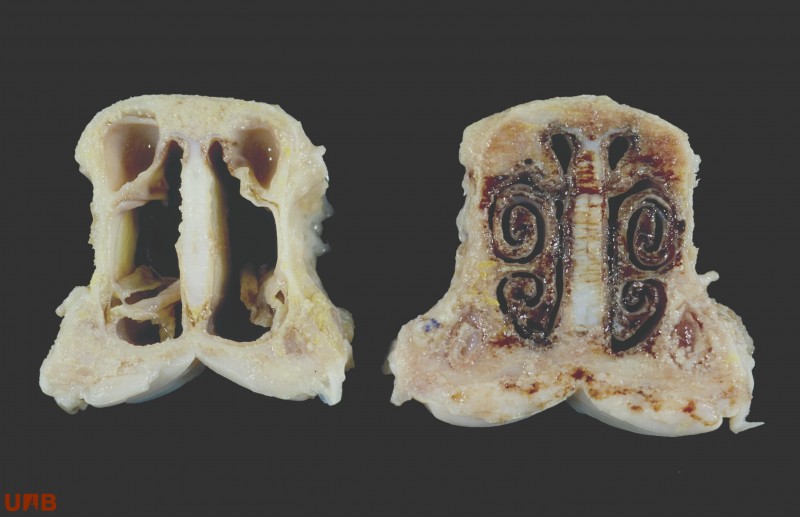
The morphologic diagnosis of atrophic rhinitis is ideally done evaluating a cross section of the skull at the level of the 1st upper premolar (or the labial commissure) that allows evaluating the atrophy of the turbinates. The image on the left corresponds to a pig with a normal turbinate morphology, on the right a clear case of atrophic rhinitis.


1 comment(s)
Comments from LinkedIn Swine Health, Nutrition and Production Professionals group:
Marco Sensi
Medico Veterinario presso Istituto Zooprofilattico Sperimentale dell’Umbria e delle Marche
In Central Italy, I’m looking a strong recrudescence of this pathology expecially in finishing units were are bred imported pigs from other EU countries.
Amber DeClercq
Graduate Research Assistant at Iowa State University College of Veterinary Medicine
Thank you for the information, as it is related to my thesis project.
Keith Wilson
Director of Swine Business Unit at Newport Labs
We used to vaccinate all pigs for Atrophic Rhinitis. Now, we don’t vaccinate any. It shouldnt be too surprising that incidence may be increasing.
Frederic PELENC
Veterinaire Responsable Technique Terrain France Filière Porcine chez VETOQUINOL SA France
Effectively, the vaccination for Atrophic Rhinitis had decrease during the last 10 years. Even though, I remember situations where, even if sows were well vaccinated, we had a high % of pigs with atrophy and malformation of nasal turbinates at slaughterhouse (without any clinical sign in the farm!!). In those situations, what was the part(or the weight) of P. multocida dermonecrotic toxin and the weight of B. bronchiseptica? Does any one have already experimented an eradication program for Bordetella in a farm with a high level of biosecurity?